This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commerci

PDF) Synthesis and SAR of sulfonyl- and phosphoryl amidine compounds as anti-resorptive agents | Sukbok Chang - Academia.edu
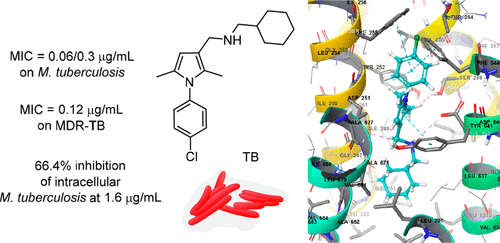
Improving the Potency of N-Aryl-2,5-dimethylpyrroles against Multidrug-Resistant and Intracellular Mycobacteria.,ACS Medicinal Chemistry Letters - X-MOL

PDF) Benzo-fused lactams from a diversity-oriented synthesis (DOS) library as inhibitors of scavenger receptor BI (SR-BI)-mediated lipid uptake

Discovery of Potent Selective Nonzinc Binding Autotaxin Inhibitor BIO-32546 | ACS Medicinal Chemistry Letters

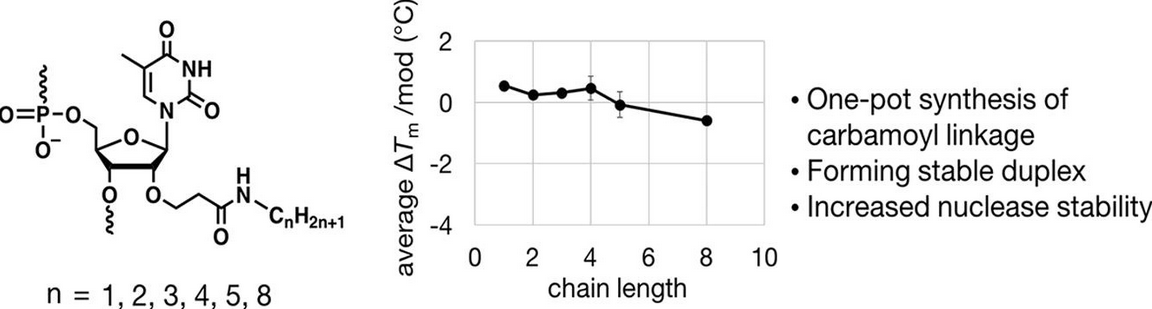
Development of Chimeric Molecules That Degrade the Estrogen Receptor Using Decoy Oligonucleotide Ligands | ACS Medicinal Chemistry Letters